Tumor Mutational Burden (TMB) Analysis
TMB represents the total number of mutations per coding area of a tumor genome, which is calculated following genomic sequencing of tumor DNA. The value of TMB has been found to be correlated with the efficacy of some anti-PD1/PD-L1 immunotherapies in certain tumor types.
NovoPM™ 2.0-TMB Algorithm
TMB is calculated based on the coding DNA sequence (CDS) regions included in the NovoPM™ panel (approximately 1.4 Mb).
Somatic mutations are identified by VarScan2.
The following mutations are excluded from the calculation of TMB:
- Germline mutations
- Low frequency mutations (Threshold: SNV MAF 1.5%; InDel MAF 3%)
- Synonymous mutations
- Repeat regions
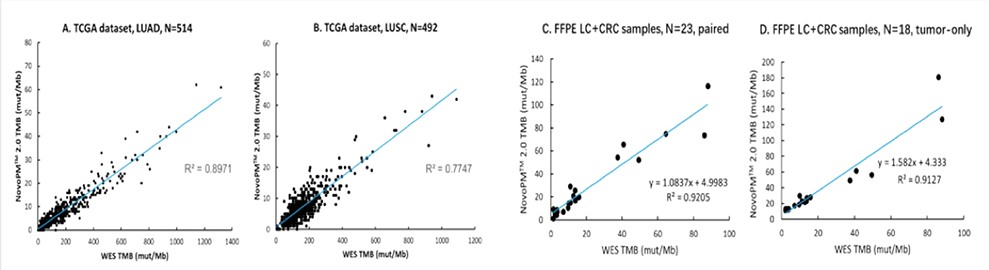
Figure 1
NovoPM™ 2.0-TMB is an alternative to TMB calculated from whole exome sequencing (WES) as there is a strong linear correlation between the two as shown in Figure 1.
Figure 1. NovoPM™ 2.0‒TMB shows strong linear correlation with WES‒TMB for two TCGA lung cancer datasets (A and B) and two Novogene in-house datasets from FFPE samples (C. paired normal-tumor samples and D. tumor-only samples). LUAD: Lung Adenocarcinoma; LUSC: Lung Squamous Cell Carcinoma; LC: Lung Cancer.
Blood Tumor Mutational Burden (bTMB) Analysis
Blood tumor mutational burden (bTMB) represents the total number of somatic mutations per coding area of a tumor genome calculated through the genomic sequencing of circulating tumor DNA (ctDNA), an alternative source of diagnostic material for patients with inadequate tissue samples. The value of bTMB has been found to correlate with the efficacy of anti-PD-L1/PD-1 immunotherapies in some tumor types.
NovoPM™ 2.0-bTMB Algorithm
bTMB is calculated based on the coding DNA sequence (CDS) regions included in the NovoPM™ panel (approximately 1.4Mb).
Somatic mutations are identified by VarScan2.
The following mutations are excluded from the calculation of bTMB:
- Germline mutations
- Low frequency mutations (Threshold: SNV MAF 1%; InDel MAF 1.5%)
- Synonymous mutations
- Repeat regions

Figure 2
NovoPM™ 2.0-bTMB has a strong linear correlation with the results calculated from our NovoPM™ 2.0-TMB. It also shows high reproducibility as presented in Figure 2.
Figure 2. Performance validation of NovoPM™ 2.0-bTMB. A. Correlation between TMB and bTMB measured by NovoPM™ 2.0 from 48 paired tissue-blood samples. X-axis: bTMB result from NovoPM™ 2.0-bTMB; Y-axis: TMB result from NovoPM™ 2.0-TMB; B. Reproducibility of NovoPM™ 2.0-bTMB.
Microsatellite Instability (MSI) Analysis
MSI is the condition of genetic hypermutability (predisposition to mutation) that results from impaired DNA mismatch repair (MMR). FDA approval of Keytruda® for MSI-high (MSI-H) solid tumors in May 2017, Opdivo® for MSI-H colorectal cancer in August 2017 and Opdivo® together with Yervoy ® for MSI-H colorectal cancer in July 2018 showed that MSI is a predictive biomarker for these anti-PD1 immunotherapies.
NovoPM™ 2.0-MSI Algorithm
To analyze tumor samples’ microsatellite status with NovoPM™ 2.0, an MSI score algorithm was developed in-house with a training set of 35 paired tumor/normal samples with known MSI status (measured by a PCR-based method, the current gold standard in clinical testing for MSI status) and then validated with a validation set of 52 such samples.
The test covered a total of 84 mononucleotide microsatellite loci and the significance of repeat length difference between tumor sample and the paired normal sample was used to determine the MSI status .
When the threshold for the MSI score was set at 0.1, the algorithm achieved 100% concordance with the PCR results (Figure 3).
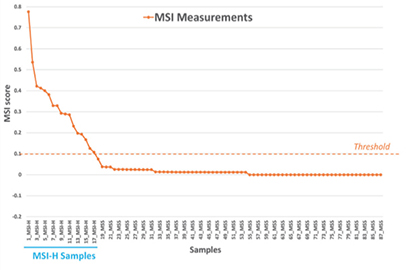
Figure 3
Figure 3. NovoPM™ 2.0‒MSI scores developed and validated with 87 colorectal cancer FFPE samples including 17 MSI-H and 70 MSS. X-axis shows the sample ID and Y-axis shows the MSI score.
