Leveraging Next-Generation Sequencing to Combat Drug Resistance in Colorectal Cancer
Introduction
Colorectal cancer (CRC) remains a significant challenge in oncology, with drug resistance posing a formidable obstacle to effective treatment. Recent advancements in next-generation sequencing (NGS) technologies have provided unprecedented insights into the molecular mechanisms underlying drug resistance, paving the way for innovative therapeutic strategies. In a seminal study published in Nature Cancer on January 30, 2024, the study by Professor Chen Quan’s team identified LGR4 as a key regulator of ferroptosis sensitivity in CRC. This paper explores the transformative potential of NGS in unravelling the intricate genomic landscape of CRC and identifying novel targets to overcome drug resistance.
Background
Despite advances in CRC therapy, acquired drug resistance remains a major cause of treatment failure and mortality. Chemotherapy regimens often include combinations of drugs targeting specific molecular pathways; however, tumours frequently develop resistance, leading to disease progression. Ferroptosis, an iron-dependent form of regulated cell death, has emerged as a promising therapeutic avenue in CRC. Recent studies have implicated Leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4) in modulating ferroptosis sensitivity, highlighting its potential as a therapeutic target. Understanding the complex interplay between molecular alterations driving drug resistance and ferroptosis regulation necessitates comprehensive genomic profiling facilitated by NGS techniques.
Materials and Methods
The study commenced with the establishment of a biobank of colorectal cancer patient-derived organoids (PDOs) from clinical samples of 40 patients, which were then cultured and treated with standard chemotherapeutic agents like 5-FU, cisplatin, irinotecan (SN-38), and combinations like FOLFOX and FOLFIRI. Following treatment, PDOs exhibited variable drug sensitivities, which were assessed through IC50 measurements. To decipher the mechanisms of drug resistance, comprehensive transcriptomic analyses were performed via transcriptome sequencing provided by Novogene, comparing drug-resistant and drug-sensitive PDOs. This revealed overactivation of the Wnt/β-catenin signaling pathway, particularly involving LGR4, in drug-resistant PDOs.
To investigate the role of LGR4 in mediating drug resistance, LGR4-positive and negative cells were isolated from PDOs using fluorescence-conjugated antibodies and sorted via flow cytometry. The drug resistance phenotype was further confirmed through comet assays, which showed less DNA damage in LGR4-positive cells compared to LGR4-negative ones when treated with chemotherapy drugs. A knockout (KO) of LGR4 in tumour organoids led to decreased Wnt pathway activity and increased cell death, indicating LGR4’s pivotal role in conferring drug resistance.
The study then explored the therapeutic potential of a monoclonal antibody against LGR4 (LGR4-mAb), hypothesizing it could inhibit LGR4’s interaction with its ligands and thereby sensitize the cancer cells to chemotherapy. To test this, LGR4-mAb was developed by immunizing mice with synthesized peptides and screened for antibody production. Effective clones were identified and tested for their specificity and efficacy in inhibiting colony formation in CRC cell lines and PDOs.
Subsequent in vitro treatments of CRC PDOs with LGR4-mAb in the presence of chemotherapeutic agents revealed significant reductions in cell viability and increased rates of apoptosis and ferroptosis, especially in drug-resistant PDOs. LGR4-mAb treatment alone, or in combination with chemotherapy, demonstrated that the antibody could overcome chemoresistance by inhibiting the Wnt/β-catenin pathway and promoting ferroptosis.
To evaluate the in vivo efficacy, the study utilized a xenograft model where treated PDOs were implanted in nude mice and subjected to various treatment regimes. LGR4-mAb, both alone and in combination with chemotherapy, significantly reduced tumour sizes and improved survival rates, illustrating the potential of LGR4-mAb to enhance chemosensitivity and induce ferroptosis in vivo.
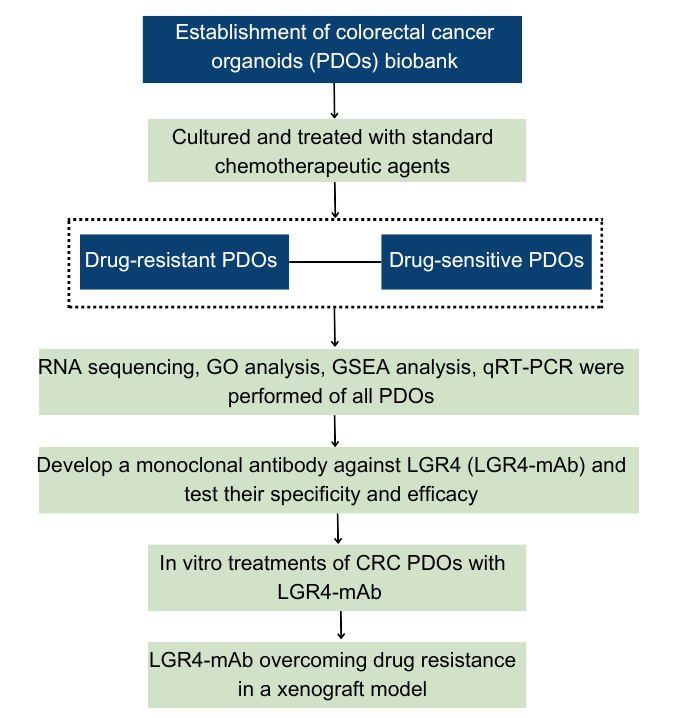
Fig. 1 Research workflow
The research pipeline (Fig. 2) concluded with comprehensive molecular analyses and survival studies correlating high levels of LGR4 and SLC7A11 expression with poor prognosis in colorectal cancer, highlighting the clinical relevance of targeting the LGR4-Wnt signalling pathway to combat drug resistance and induce ferroptosis in cancer cells. This detailed pipeline effectively demonstrates a rigorous and multi-faceted approach to understanding and potentially overcoming drug resistance in colorectal cancer through targeted therapeutic strategies.
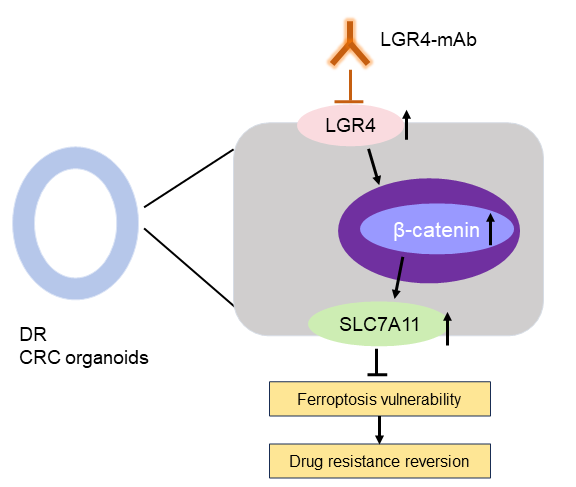
Fig. 2 A schematic diagram illustrating that LGR4-mAb sensitizes DR CRC organoids, adapted from [1].
Conclusion
To conclude, this case study successfully demonstrates how targeting the LGR4-Wnt-β-catenin pathway can overcome drug resistance in colorectal cancer. Using a monoclonal antibody to inhibit LGR4 effectively sensitized resistant CRC organoids to chemotherapy by inducing ferroptosis. These promising results, validated in both laboratory and animal models, pave the way for potential clinical applications to enhance treatment efficacy in CRC patients.
The Role of Next-Generation Sequencing
Next-generation sequencing (NGS) technologies allows for the simultaneous analysis of multiple genes and pathways, facilitating the identification of genetic alterations associated with drug resistance. Whole-genome sequencing (WGS), whole-exome sequencing (WES), and transcriptome sequencing approaches have been employed to delineate the genomic landscape of CRC, uncovering recurrent mutations, copy number alterations, and gene expression patterns associated with drug resistance.
Novogene has accumulated extensive experience in DNA and RNA library preparation, sequencing, and bioinformatics analysis across numerous species. If you have personalized requirements, please make a comment on the request for quote form, and we can discuss in detail.
Reference
[1] Zheng, H., Liu, J., Cheng, Q., Zhang, Q., Zhang, Y., Jiang, L., Huang, Y., Li, W., Zhao, Y., Chen, G., Yu, F., Liu, L., Li, Y., Liao, X., Xu, L., Xiao, Y., Zheng, Z., Li, M., Wang, H., . . . Chen, Q. (2024). Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance. Nature Cancer. https://doi.org/10.1038/s43018-023-00715-8
[2] More Evidence that Cellular ‘Death by Iron’ Could Be Promising Avenue. (n.d.). Memorial Sloan Kettering Cancer Center. https://www.mskcc.org/news/more-evidence-death-iron-could-be-promising-avenue-cancer-treatment
