Clinical Whole Exome Sequencing Service
Novogene offers CLIA-validated Whole Exome Sequencing (WES) for investigation or diagnoses of genetic variations underlying cancers, Mendelian diseases, and complex human disorders.
Whole Exome Sequencing is gaining popularity as a viable and cost-effective alternative to Whole Genome Sequencing: WES targets all protein-coding regions (~1% of the whole genome) responsible for 85% of all disease-causing mutations.
Service Features
- Starting Materials: gDNA, Whole Blood, Saliva, Buccal Swab, and FFPE samples
- Applications: Germline Variants and Somatic Variants
- Sequencing Platform: Illumina NovaSeq 6000 platform
- Sequencing Coverage: 50X or 100X
- Turnaround Time: 2 Weeks (10 Working Days)
- Quality Assurance: Performed by Licensed Personnel in CLIA-certified Laboratory
- Deliverables: FASTQ, BAM, VCF Files, and Medical Report (optional)
- Data Transfer: AWS and Free Data Uploading Services
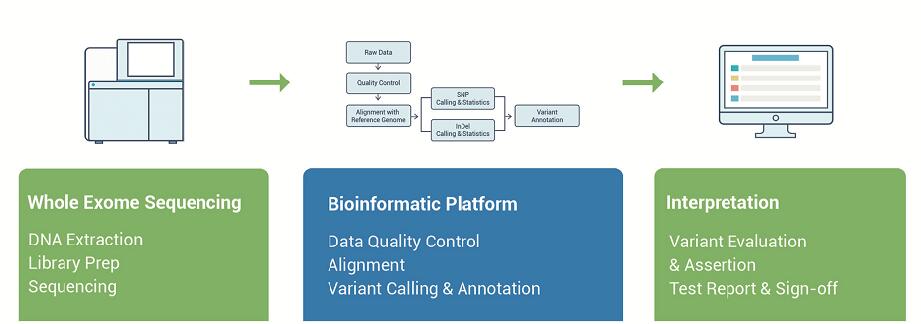
Clinical WES Workflow
Performance Metrics
| Validated Mean Sequencing Coverage | 103x | |
| Average Raw Data | 13.78 G | |
| Base Pairs Covered at ≥20x | ≥ 94% | |
| Coverage Uniformity | ≥ 90% | |
| Mapping Rate | ≥ 97% | |
| Repeatability | SNVs | ≥ 98% |
| Indels | ≥ 88% | |
| Sensitivity | SNVs | ≥ 98% |
| Indels | ≥ 90% | |
| Specificity | SNVs | ≥ 99% |
| Indels | ≥ 98% | |
Download Brochure to Learn More
Contact Us / Request Quote

Novogene Corporation Inc.
![]() 8801 Folsom Blvd #290, Sacramento, CA 95826
8801 Folsom Blvd #290, Sacramento, CA 95826
Copyright©2011-2020 Novogene Corporation
All Rights Reserved. Information and specifications are subject to change at any time without notice.
